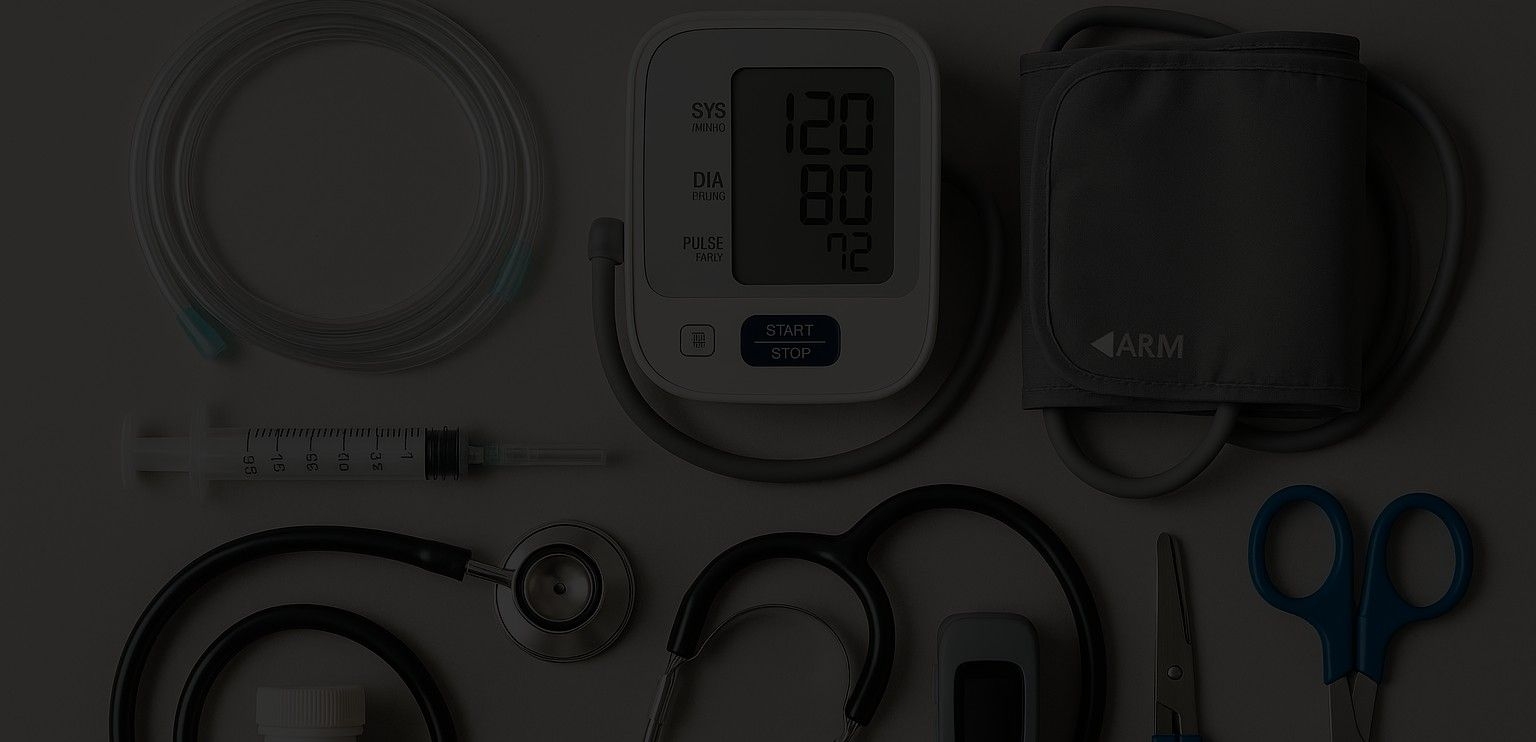
The Complete Guide to Shipping Non-Temperature-Sensitive Medical Devices


Shipping non-temperature-sensitive medical devices globally requires careful planning and logistics. These products must move through;
- Regulatory compliance requirements
- Careful handling procedures
- Accurate documentation and labeling
Each step affects delivery time, compliance risk, and freight cost.
This guide will help you understand how to ship these medical products safely, compliantly, and efficiently across borders.
What Are Non-Temperature-Sensitive Medical Devices?
Common Types and Use Cases
Non-temperature-sensitive medical devices are healthcare products that do not require cold-chain transport and remain stable at room temperature.
Even though they don’t need temperature control, these devices still require special care because they are often:
- Fragile
- High-value
- Sterile
- Precision-engineered
Non-Temperature-Sensitive Medical Devices: Examples
Surgical instruments
Such as scalpels, scissors, and forceps used in operating rooms daily
Orthopedic implants
Like screws, plates, and rods that require exact positioning and structural integrity
Diagnostic equipment
Like ECG machines, glucometers, and stethoscopes used in patient monitoring
Hospital furniture
Such as beds, trolleys, and exam tables—bulky but critical for patient care
Mobility aids
Like crutches, walkers, or wheelchairs used in rehabilitation or elderly care settings

Differences from Temperature-Controlled Devices
Temperature-controlled products like vaccines and biologics need cold-chain transport (2–8°C). Non-temperature-sensitive devices do not. However, both still follow strict regulatory rules.
- Can be safely stored and transported at room temperature (15–25°C)
- Are more resilient to minor delays or environmental shifts during transit
- Have longer shelf lives and broader handling tolerances
- Prioritize physical protection (shock, vibration, sterility) over thermal protection
Why Proper Shipping Matters for Medical Devices
Shipping medical devices must be precise. Errors can cause customs delays, rejects, or damaged products.
- Customs holds from missing or mismatched paperwork
- Damage from poor handling
- Blocked entry due to incorrect packaging or missing certifications
Timely, compliant, and secure delivery builds trust with healthcare buyers and supports long-term success.
Packaging Essentials
Packaging Requirements and Best Practices
Protective Materials
Effective packaging is your first line of defense against damage. Medical equipment can be vulnerable to vibration, drops, or compression—especially during multi-modal shipments. Depending on the product:
- Use foam inserts or molded trays to secure sensitive equipment
- Choose double-walled corrugated boxes for maximum stacking strength
- Apply shock indicators or tilt sensors for fragile, high-value instruments
- Stack and wrap shipments on standardized pallets for safe forklift handling
Good packaging protects goods and meets hospital or clinic expectations.
Sterile Packaging
If your devices are sterile, like surgical drapes or disposable scalpels, packaging becomes even more critical. Contamination—even from the air—can render the product unusable.
- Use Sterile Barrier Systems (SBS) compliant with ISO 11607 standards
- Pack within controlled environments, such as cleanrooms
- Clearly label outer cartons with "Sterile", expiry dates, and handling instructions
Sterility isn’t negotiable—it’s a regulatory requirement and a patient safety issue. Your packaging must preserve integrity from origin to delivery.
Labeling and Instructions for Medical Equipment Logistics
Clear, compliant labeling ensures a smoother customs process and safe last-mile delivery. Labels act as both regulatory identifiers and logistical instructions for handlers across borders.
Each shipment should include:
- Product name, SKU/part number, and clear handling instructions (e.g., “Fragile – Do Not Drop”)
- Manufacturer name, country of origin, and contact details
- If sterile, expiry dates and sterility indicators
- Barcodes, serial numbers, or UDI codes for tracking and regulatory traceability
Incorrect labeling can cause customs holds. It can also lead to misdeliveries or product recalls.
Freight Comparison
Freight Options: Air vs. Ocean
When to Choose Air Freight
Air freight is often chosen for:
- Time-sensitive shipments such as emergency hospital restocking
- High-value, low-volume equipment (e.g., diagnostic monitors, implants)
- Deliveries to remote or landlocked destinations
Air freight usually costs more than ocean freight. However, it offers fewer handling points and faster transit for smaller loa
When to Choose Ocean Freight
Ocean freight is ideal when:
- You’re shipping large or heavy equipment like exam tables or patient beds
- You have more flexible timelines
- You're sending bulk orders across borders
FCL (Full Container Load) offers greater security, while **LCL (Less than Container Load) **can reduce cost for small or moderate shipments. However, LCL may involve additional handling at depots, so packaging must be especially robust.
Customs Compliance
Documentation and Customs Compliance
Essential Documents Checklist
Proper documentation is critical for customs clearance and regulatory compliance. Every document must match across product details, values, and quantities. Even small discrepancies can cause long delays or inspections.
Required documents for medical device shipments;
- Commercial Invoice — full product description, HS codes, and declared values
- Packing List — weights, quantities, dimensions, and packaging details
- Bill of Lading (B/L) or Air Waybill (AWB) — transport contract and tracking reference
- Proforma Invoice — used when pre-approval or licensing is required
- Certificate of Origin — proves manufacturing country for trade and duty purposes
tip: always ensure part numbers, product names, and serial data match across all paperwork.
Regulatory Certifications
Medical devices are often subject to strict import regulations. Depending on your destination:
- Secure FDA 510(k) or De Novo approval for the U.S.
- Display valid CE markings for the EU
- Follow Health Canada guidelines for imports into Canada
- Include test reports, declarations of conformity, or registration numbers for Latin America (e.g., ANVISA in Brazil)
Staying updated on regulatory changes in each region is vital—especially for exporters scaling globally.

HS Codes and Tariff Classifications
Every item must be properly classified under the Harmonized System (HS). Use the most specific codes available. For example, classifying orthopedic screws correctly avoids misclassification under general surgical tools. Errors here can cost thousands in overpaid duties—or worse, shipment holds. This determines:
- Applicable duties and taxes
- The likelihood of customs inspections
- Eligibility for trade agreements
Insurance and Security for Medical Shipments
Types of Coverage
Depending on the destination, carrier, and cargo value, insurance should be customized. Options include:
- All-risk coverage for sensitive or high-value shipments
- Named-perils policies for cost control with basic protection
- Third-party liability insurance required by some healthcare providers or ministries
Always insure shipments at their declared commercial value and understand what your policy excludes—especially for LCL shipments.
Tamper-Proofing and Theft Prevention
Medical shipments are often targeted due to their value and demand. To reduce risk:
- Use tamper-evident tape and lock
- Avoid product-specific branding on outer boxes
- For high-risk areas, consider GPS or IoT-enabled tracking
- Partner with freight forwarders experienced in medical cargo security
Final Delivery and Last-Mile Logistics
The final leg of your shipment is just as important as the transcontinental journey. If it’s not handled well, everything before it may go to waste.
Ensure your logistics partner can offer:
- Full end-to-end tracking, including customs events
- Proof of delivery (POD) via signature, photo, or timestamp
- Pre-scheduled appointments for delivery to hospitals or clinics
- On-site coordination for unloading using lifts, ramps, or pallet jacks
Failing to manage the last mile can result in refused shipments or extra handling fees.
How iContainers Supports Your Medical Device Shipping
Shipping non-temperature-sensitive medical devices requires precision, regulatory knowledge, and logistical expertise. While these items may not demand temperature control, they do require careful handling, robust documentation, and secure packaging —and that’s where iContainers comes in.
At iContainers, we help SMEs simplify international shipping through:
- End-to-end compliance support for medical logistics, including FDA, CE, and other market-entry requirements
- Customs-ready documentation and digital tools to avoid costly delays or rejections
- Tailored freight solutions—from cost-effective LCL for small batches to FCL for large-scale hospital tenders
- Reliable global carrier partnerships to ensure timely and secure delivery
- Transparent pricing and full visibility with our online platform and shipment tracking features
Whether you’re shipping orthopedic implants to a clinic in Canada or diagnostic monitors to a distributor in Europe, iContainers is your logistics partner for smart, compliant, and scalable medical device exports.
Let us take the complexity out of your supply chain—so you can focus on what matters most: patient care.